Understanding Europe's Medical Device Regulation

1:12:45
The New Medical Device Regulation (MDR) - Webinar

1:05:09
Mastering the Regulatory Landscape for AI/ML- enabled SaMD

35:28
THE RIGHT Way to Register Your Trademark Without Cutting Corners

57:57
MLRN - Using beneficial ownership data for large-scale risk assessment in public procurement

58:34
Documentation Deconstructed: Understanding the Technical file

1:15:56
Power System Oscillations in High Renewable Power Systems: One Example Event and Guide Review
57:47
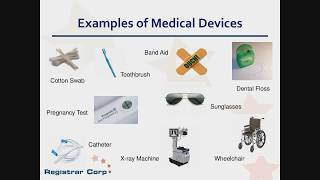
FDA 101 for Medical Devices

1:11:19