FDA 101 for Medical Devices

1:32:39
Medical Devices: Overview of US FDA regulatory process

49:08
Medical Devices 101: An Entry Level Overview of the FDA

34:08
Stepping Inside the World of Contract Manufacturers for Medical Devices
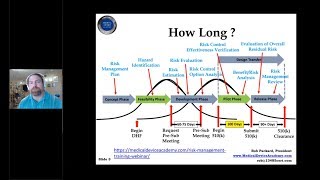
1:34:30
How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe Hage

4:01:28
U.S. FDA Label Regulations for the Korean Cosmetic Industry (Korean Webinar)
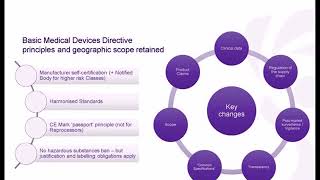
1:06:28
Medical Devices Regulation Training

1:03:43
Procurement and Supply Chain Management for Medical Devices, Diagnostics, and Equipment (MDDE)

1:39:26