The New Medical Device Regulation (MDR) - Webinar
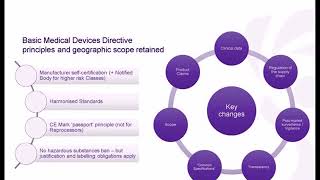
1:06:28
Medical Devices Regulation Training
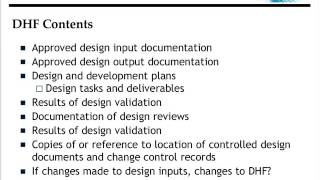
1:02:57
FDA Quality Systems Regulation Requirements - Regulatory Documents Explained
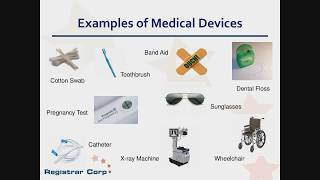
57:47
FDA 101 for Medical Devices

1:26:42
Harvard i-lab | Understanding Medical Device Development

1:39:26
Design Controls - Requirements for Medical Device Developers

45:19
CE Marking - practical approach guide

1:12:06
Medical Devices - ISO 14971 : Risk Management

1:25:43