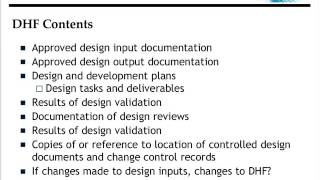
Regulatory Documents Explained - DHF, DMR, DHR and TF
1:02:57
FDA Quality Systems Regulation Requirements - Regulatory Documents Explained

58:34
Documentation Deconstructed: Understanding the Technical file

59:53
Medical Device Design Control

1:39:26
Design Controls - Requirements for Medical Device Developers

17:31
Design Control for Medical Devices - Online introductory course

1:10:24
FDA Requirements for Device Labeling

1:07:19
Supplier Evaluation & Assessment How to Meet FDA QSR & ISO 13485 Requirements

1:12:45