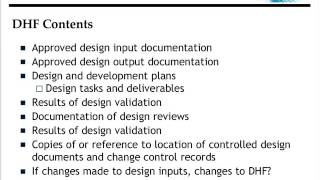
Regulatory Documents Explained - DHF, DMR, DHR and TF

1:05:37
Managing the Medical Device Supply Chain

58:34
Documentation Deconstructed: Understanding the Technical file
1:02:57
FDA Quality Systems Regulation Requirements - Regulatory Documents Explained

1:10:24
FDA Requirements for Device Labeling

1:19:59
Requirements Contents and Options : The 510k Submission

1:08:18
How FDA Trains its Investigators to Review CAPA and What You Should Do to Prepare

1:12:45
The New Medical Device Regulation (MDR) - Webinar

17:07