Auditing Analytical Laboratories for FDA Compliance

1:13:50
21CFR Part 58 The Good Laboratory Practices GLP Regulation

1:04:54
Complaint Handling in Compliance with FDA and ISO Regulations

59:48
Supplier and Internal Auditing

22:01
Bioanalytical Method Validation of a Small Molecule in a Surrogate Matrix by LC-MS/MS
1:28:00
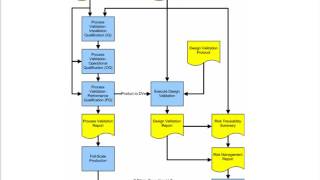
Process Validation for Medical Device Manufacturers

4:00:37
4 Hours Chopin for Studying, Concentration & Relaxation

2:22:09
Kızılcık Şerbeti 100. Bölüm @showtv

1:12:03