21CFR Part 58 The Good Laboratory Practices GLP Regulation

1:31:06
The FDA Drug Development Process: GLP, GMP and GCP Regulations

53:06
GLP webinar

13:01
أسعار المواشي مباشرمن سوق حاسي بحبح ولاية الجلفة الخميس 10أكتوبر 2024
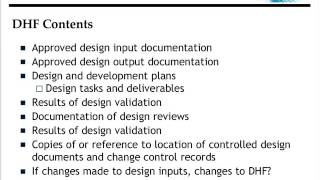
1:02:57
FDA Quality Systems Regulation Requirements - Regulatory Documents Explained

1:51:49
Auditing Analytical Laboratories for FDA Compliance

41:47
MACSQuant® Tyto® Cell Sorter:Validating a fluorescence sorter for infectious diseases [WEBINAR]

58:20
Think Fast, Talk Smart: Communication Techniques

1:09:35