FDA 101 for Medical Devices

49:08
Medical Devices 101: An Entry Level Overview of the FDA

1:19:08
Unpacking FDA Food Labeling and Ingredient Regulation: What Companies Must Know Before Exporting

1:19:55
Thin Green Line 2.0 at PIELC25

1:32:39
Medical Devices: Overview of US FDA regulatory process
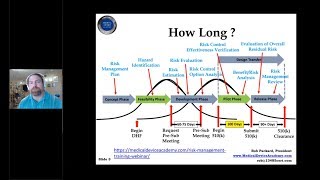
1:34:30
How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe Hage

1:30:39
Unwrapping FDA's MoCRA Labeling Regulations

34:08
Stepping Inside the World of Contract Manufacturers for Medical Devices

1:00:09