Work Done in Quasistatic (Reversible) Compression and Expansion of a Gas in a Piston (W = - P dV)
4:16
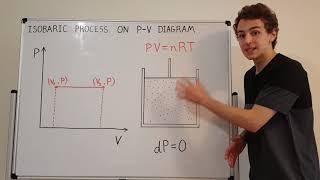
Quick Explanation of Isobaric Process on P-V Diagram
16:00
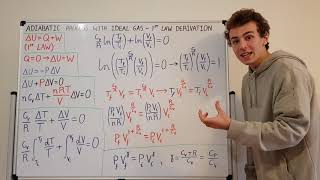
Adiabatic Process with Ideal Gas - First Law of Thermodynamics Derivation (Integration, Natural Log)

7:43
Quantitative Description of Isobaric (Constant Pressure) Process with Ideal Gas

10:10
Isochoric/Isovolumetric (Constant Volume) Process on P-V Diagram

9:09
Adiabatic Process with Ideal Gas - Work Derivation (Integration, Area Under Curve, Exponent, Gamma)

4:39
Calculate Work for Reversible and Irreversible Expansion/Compression

20:17
PV Diagrams, How To Calculate The Work Done By a Gas, Thermodynamics & Physics

13:00