Why only three batches for validation

2:39
Purpose of a media fill test in sterile production

3:29
Three Consecutive Batches for Validation | Why Three Batches are Considered in Validation

12:46
ICH guidelines Quality

10:14
Equipment Validation I Pharmaceutical Industry l DQ IQ IQ PQ

11:25
Process Validation | PV and its Life cycle approach

15:40
AREA CLASSIFICATION, Clean room classification.
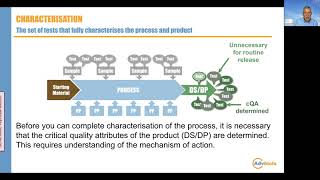
22:32
Process performance Qualifications (PPQ) for cell-based products

6:14