USFDA – 483 form in Pharmaceutical industry l 15 Important Question and answers l Warning letter .

3:37
Schedule-M l Revised Schedule-M 2023 l drugs and cosmetics act 1940 l Pharmaceutical industry

15:10
Literatür Taraması Nasıl Yapılır

4:39
How to Respond to FDA 483 Observations: Key Considerations and Best Practices

10:24
Regulatory Affairs Explained Episode 1: FDA, Application Types, Regulatory Pathways & More
18:25
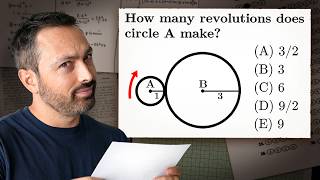
The SAT Question Everyone Got Wrong

4:25
What is 482 form|483 form|484 form|EIR report|NAI|OAI|VAI.

10:25
QMS in Pharmaceutical industry l Quality Management system in Pharma Industry l Question & answers

22:38