
57:39
Handling Suspected Fraud and Data Fabrication in Clinical Studies

1:07:46
Development and Delivery of Pharmaceutical Products (CMC) - MaRS Best Practices

59:41
Leveraging Interim Analyses to Optimize Late Phase Clinical Trial Decision Making
2:03:01
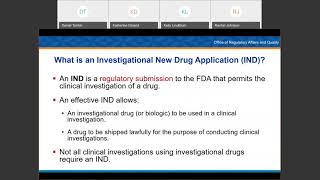
Investigational New Drug Workshop

55:45