Introduction to Analytical Quality by Design (AQbD) principles
1:02:17
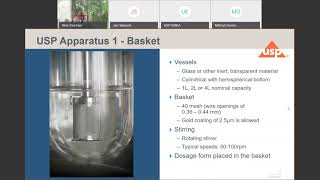
Qualification of Dissolution Testers USP Performance Verification Test (PVT)

58:50
Validation, Verification, & Transfer of Analytical Methods – USP General Chapters 1224, 1225 & 1226

13:46
How Blake Scholl Built The First Independent Supersonic Jet

1:01:58
QbD in Biologics Drug Product Development and Manufacturing

50:51
Strategies for HPLC Method Development - Webinar Recording

1:01:05
A-Cell: Generation of QTPP, Risk Assessment and Critical Quality Attribute Identification

59:58
The Analytical Procedure Life Cycle – Where does the journey go with General Chapter 1220

49:27