Design Controls - Requirements for Medical Device Developers

1:08:12
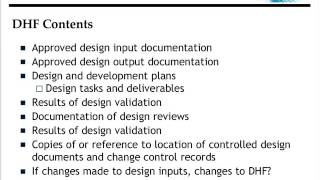
Process Validation Principles and Protocols for Medical Devices
1:02:57
FDA Quality Systems Regulation Requirements - Regulatory Documents Explained

37:27
Determining User Needs for Your Medical Device

1:10:24
FDA Requirements for Device Labeling

59:10
Design Controls 101 and Implementation Best Practices - Galen Data
1:28:00
Process Validation for Medical Device Manufacturers

1:25:47
EU Postmarket Surveillance Requirements for Medical Devices

1:05:37