How to select a Dissolution medium for IR product with BCS- I Drug substance?

15:47
Dissolution method development for Immediate Release (IR) drug product

26:07
How to define limit for unknown, known and total impurities
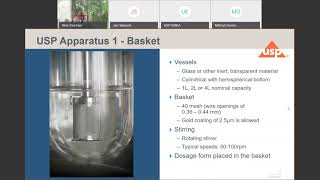
1:02:17
Qualification of Dissolution Testers USP Performance Verification Test (PVT)

7:55
Pharmacokinetics: How Drugs Move Through the Body

24:36
Out Of Specification (OOS) Investigation Phase Ia & Phase Ib (MHRA)

9:15
What Next if the Dissolution fails at S1, S2, or S3?

15:03
How to decide impurities in API & Drug Products and their release and shelf life specification

15:32