Hybridization in Carbon Dioxide, CO2 | sp, sp2 | Sigma, Pi Bonds | Lewis Structures | VSEPR Geometry

2:22
73. Explain why each electron dot structure is incorrect. | Lewis Structures | VSEPR Geometry
10:55
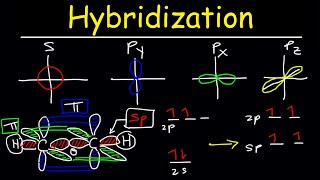
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3

6:31
VSEPR Theory and Molecular Geometry

7:26
Orbital Overlap Diagram for C2H4 (Ethene / acetylene, double bond)

8:22
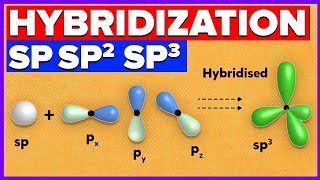
How to determine Hybridization - s, sp, sp2, and sp3 - Organic Chemistry

23:48
Exam Solutions - Electron Configuration, Periodic Table, Trends, Chemical Bonds, Lewis Structures

11:18
9.4 Sigma Bonds and Pi Bonds | General Chemistry
13:48