Complaint Handling in Compliance with FDA and ISO Regulations

1:07:19
Supplier Evaluation & Assessment How to Meet FDA QSR & ISO 13485 Requirements
1:02:57
FDA Quality Systems Regulation Requirements - Regulatory Documents Explained

1:00:35
Medical Device Complaint Handling: MDR, Reports of Removals and Corrections

1:51:49
Auditing Analytical Laboratories for FDA Compliance

10:16
IQ OQ PQ | Process Validation | Equipment Validation | Equipment Qualification | Medical Devices

10:38
The 5 most relevant changes the Medical Device Regulation MDR introduces, that you must know

1:39:26
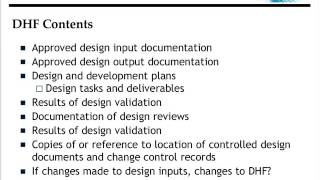
Design Controls - Requirements for Medical Device Developers

16:47