Preparing an FDA 510 (k) Submission often called 510k

38:26
How to prepare an FDA eSTAR 510(k) submission

28:21
FDA Regulations and Medical Device Pathways to Market

15:32
9 Things That Don't Make Sense After 61!
57:47
FDA 101 for Medical Devices

1:19:59
The 510(k) Submission: Requirements, Contents, and Options
1:28:00
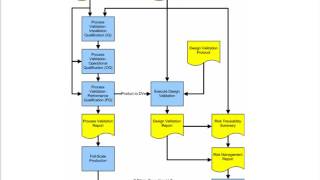
Process Validation for Medical Device Manufacturers

13:40
How long does a 510(k) submission take to get cleared?

46:45