How to prepare an FDA eSTAR 510(k) submission

7:48
Health Canada Pilot with the FDA eSTAR template

1:33:59
510(k) Project Management - Updated for 2021

14:12
Should you use an FDA eCopy or an FDA eSTAR to respond to FDA deficiencies?

1:06:28
Mastering your 510(k) submission process

1:36:15
Software Validation Documentation for FDA 510(k) pre-market notification submission
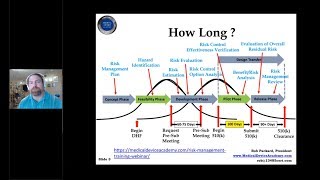
1:34:30
How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe Hage

1:14:02
Update on the NCVS Instrument Redesign Additional Findings from the National Field Test and Plans

22:59